Look, Feel, & Fit
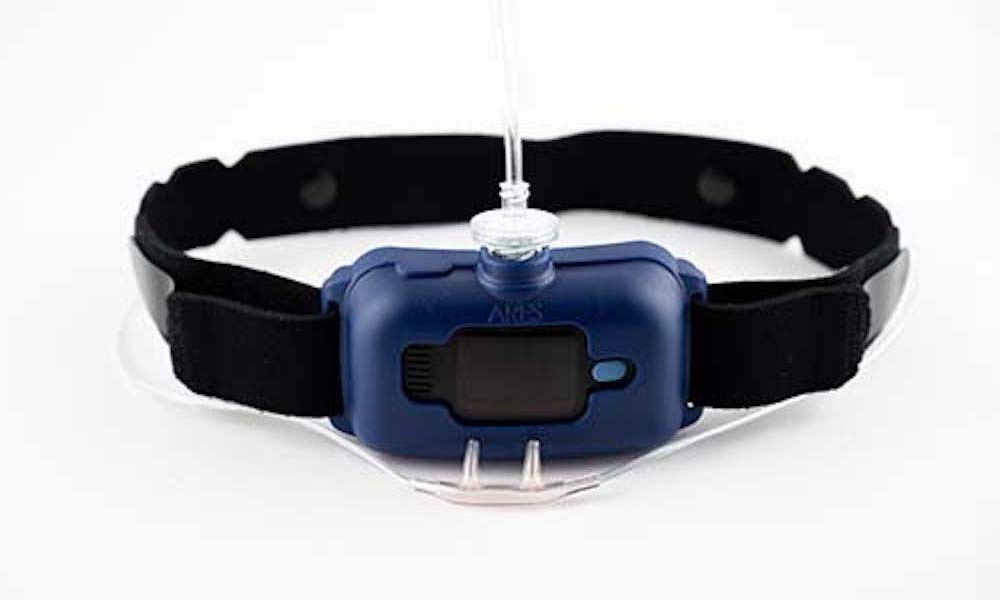
The ARES 620 consists of a nasal cannula and a device that is worn on the forehead and held in place with a headband. Most sleepers are likely to find this low-profile design minimally invasive.
Ideal For
- People with symptoms of severe obstructive sleep apnea (OSA)
- Those who find it difficult to fall asleep in unfamiliar situations
- Anyone in need of a sleep apnea test that tracks neurological metrics
Who Should Keep Looking
- People with mild OSA symptoms
- Those who are unable to sleep at least five hours per night
- Anyone whose medical history indicates the need for an in-lab sleep study
-
Price
$3,380
The Watermark Medical ARES 620 is an at-home sleep apnea test that is prescribed and provided to users by a medical professional. The testing device features a forehead sensor and a nasal cannula that track a variety of metrics that doctors can use to diagnose sleep apnea.
While sleep apnea is usually diagnosed following a sleep study at a clinic, at-home sleep apnea tests like the ARES 620 allow people to sleep in their own bed during the testing process.
To help you better understand how the ARES 620 detects sleep apnea, we’ll walk you through how it works and the metrics it tracks. We’ll also look closely at how it compares to in-lab studies.
Use this SleepApnea.org link for the most current discount on WaterMark Medical products
Shop NowWhat Is Obstructive Sleep Apnea (OSA)?
Obstructive sleep apnea is a condition that causes pauses in breathing or episodes of shallow breathing during sleep. Called apneas and hypopneas, these breathing disturbances occur when relaxed muscles and tissue in the throat obstruct a person’s airway.
OSA is common, impacting an estimated 10% to 30% of adults in the U.S. It is the most prevalent form of sleep apnea. People who smoke are more likely to have OSA, as are people with a higher body mass index. The incidence of OSA rises with age.
The apnea-hypopnea index (AHI) is a scale doctors and sleep specialists use to diagnose OSA and determine its severity. AHI score is calculated by dividing the number of nightly apnea and hypopnea events a person experiences by the number of hours they slept.
| Sleep Apnea Severity | AHI Score |
|---|---|
| Mild OSA | 5-15 |
| Moderate OSA | 15-30 |
| Severe OSA | 30 or higher |
Signs and Symptoms of OSA
Speak with your doctor if you suspect that you may have OSA. Common symptoms include, but are not limited to:
- Loud snoring that may include snorting, choking, and gasping sounds
- Frequent awakenings
- Increased nighttime urination
- Morning headaches
- Excessive daytime sleepines
How the ARES 620 Detects Symptoms of Obstructive Sleep Apnea
The standard ARES 620 consists of a forehead sensor and a nasal cannula with prongs that are inserted inside the nostrils. Together, these device components track several key metrics that collectively can help diagnose OSA:
- Pulse rate: By tracking your pulse rate, the ARES 620 can help your doctor detect heart rate changes that indicate you have experienced an apnea event.
- Blood oxygen level: The ARES 620 contains a sensor that estimates how much oxygen is present in the bloodstream. When a person with OSA stops breathing during sleep, it can lead to oxygen desaturation. A blood oxygen saturation level that dips below 90% is considered harmful. The ARES 620 provides estimates of average oxygen saturation, the percentage of time spent with an oxygen saturation of less than 90%, and the average decrease in oxygen saturation after a respiratory event.
- Sleep health: The ARES 620 combines several neurological metrics to track whether you’re asleep and how long you stay in both rapid eye movement (REM) sleep and other sleep stages. The device also includes a microphone that records snoring levels.
- Arousal indicators: Even if they’re unaware of it, people with OSA often wake up briefly following an apnea event. The ARES 620 uses metrics like pulse rate, body and head movements, and strength of breathing to track these arousals.
Types of Sleep Apnea This Device Detects
The standard ARES 620 is designed to help diagnose OSA. However, the optional effort belt provides information that can be used to detect central sleep apnea (CSA), a condition in which the brain briefly fails to send signals to the muscles that control breathing.
While people with OSA experience breathing resistance due to a physical blockage in the upper airway, those with CSA don’t make an effort to breathe during apneic episodes. The effort belt works in conjunction with the forehead sensor to identify correlations between breathing disruptions and respiratory effort.
Is This At-Home Sleep Apnea Test Approved by the FDA?
The ARES 620 has been cleared by the Food and Drug Administration as a breathing frequency monitor for adults who have received a prescription for the device.
How the Watermark ARES 620 Stacks Up Against Traditional Sleep Studies
A lab-based sleep study, known as polysomnography, is the most comprehensive method of determining if a person has sleep apnea. During an overnight stay at a sleep clinic, electrodes and other sensors attached to the body monitor a variety of sleep and health metrics that can be used to diagnose sleep disorders. Clinicians are present to observe the process and ensure accurate data collection.
At-home sleep testing devices have fewer contact points and collect less data than an in-person study. While the ARES 620 is more comprehensive than most at-home tests — particularly in its use of electroencephalography (EEG) to track brain activity — it has certain limitations. A research study involving an earlier iteration of the device showed that while it generally agreed with polysomnography in detecting severe OSA, it was less accurate at identifying mild and moderate OSA.
However, the convenience and comfort of sleeping in your own bed have made at-home tests like the ARES 620 an attractive alternative to overnight sleep studies for many people. If the results of an at-home test are inconclusive, the prescribing doctor can order additional testing in a lab-based setting.
| ARES 620 | In-Lab Sleep Study |
|---|---|
Benefits:
| Benefits:
|
Limitations:
| Limitations:
|
What You Need to Know About the Watermark Medical ARES 620
The ARES 620 is sold to medical professionals rather than the general public. If your doctor believes you would benefit from testing with the ARES 620, they will rent you the device for one or more nights. The device is reusable, though certain components, such as the nasal cannula and the headband, are disposable.
What Comes With It
If your doctor prescribes the ARES 620, they will provide you with everything needed to use it at home. They may also provide you with the ARES questionnaire, which collects information that can aid in an OSA diagnosis and ensure you’re a good candidate for at-home testing. Depending on your symptoms and medical history, they may ask you to wear the ARES 620 effort belt during testing.
Components:
- Forehead sensor
- Headband
- Nasal cannula
- ARES questionnaire (optional)
- Effort belt (optional)
Use this SleepApnea.org link for the most current discount on Watermark Medical products
Shop NowYour doctor will walk you through setting up and using the ARES 620 before providing you with the device. Further instructions, including video guides, are available online from Watermark Medical.
Sleep Data Results and Diagnosis
Your doctor will examine your test results after you have returned the ARES 620 device, and they will likely walk you through the expected timeline for a potential diagnosis.
If your doctor determines that you have obstructive sleep apnea, they may recommend CPAP therapy. In this scenario, the next step is to schedule a CPAP titration study to determine an appropriate pressure setting. Alternatively, they may prescribe an automatic positive airway pressure (APAP) machine. These devices generally don’t require lab-based titration since they automatically adjust air pressure within a set range based on the user’s breathing patterns.
What Do Customers Have to Say About the Watermark ARES 620?
Since the ARES 620 is marketed to medical professionals rather than the general public, there are limited user reviews. However, the majority of reviews report a positive experience, particularly when compared with an in-lab sleep study.
Most reviewers, though not all, found the ARES 620 easy to set up. The most common complaint among users was that the audio troubleshooting prompts startled them when they were approaching sleep. These prompts occur when there is a connectivity issue with the device’s components.
Medical Disclaimer: This content is for informational purposes and does not constitute medical advice. Please consult a health care provider prior to starting a new treatment or making changes to your treatment plan.
Still have questions?
Sleep apnea products can be confusing. If you need individualized assistance, send us an email at [email protected] with your questions and we'll help find the best fit for you.