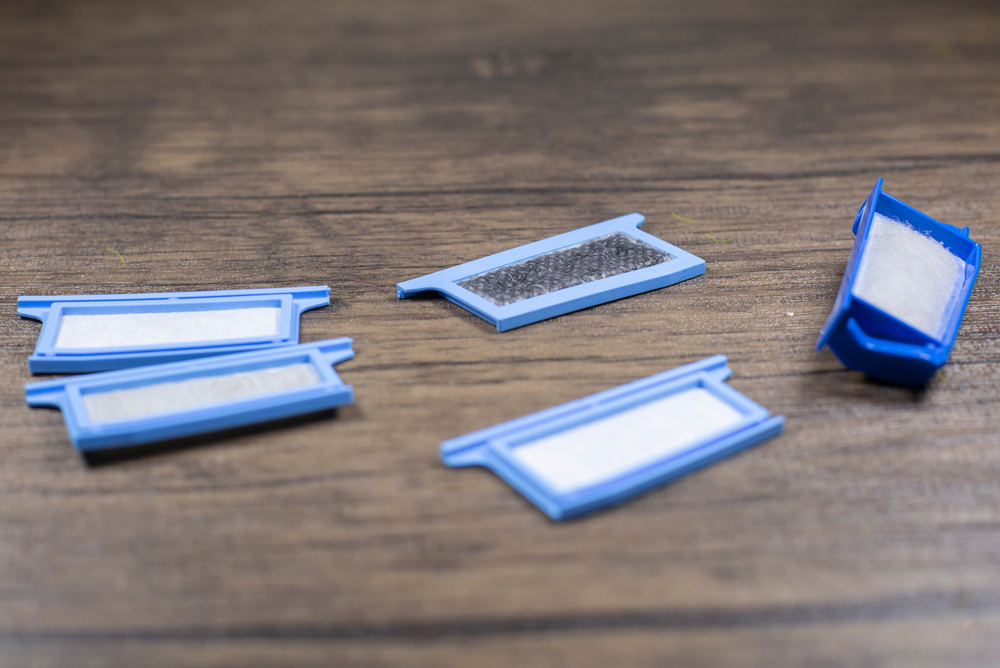
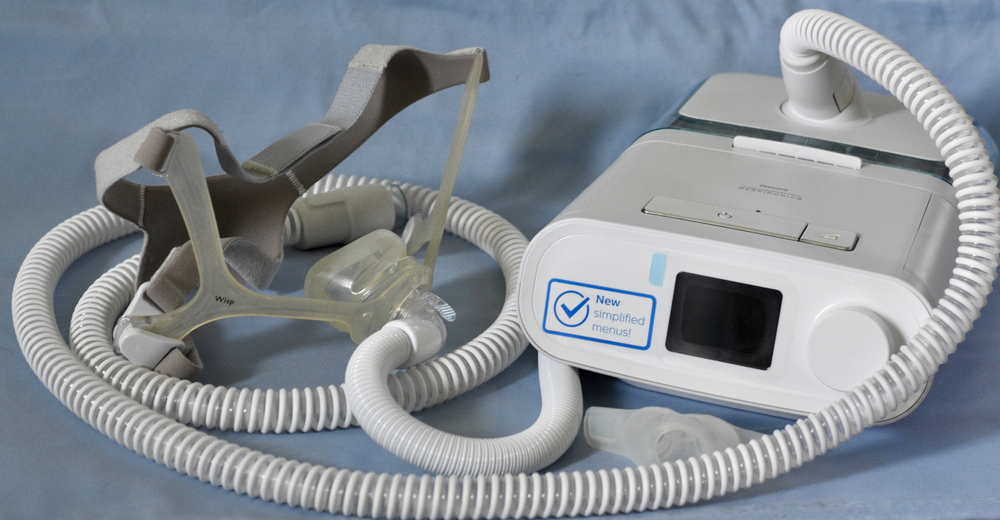
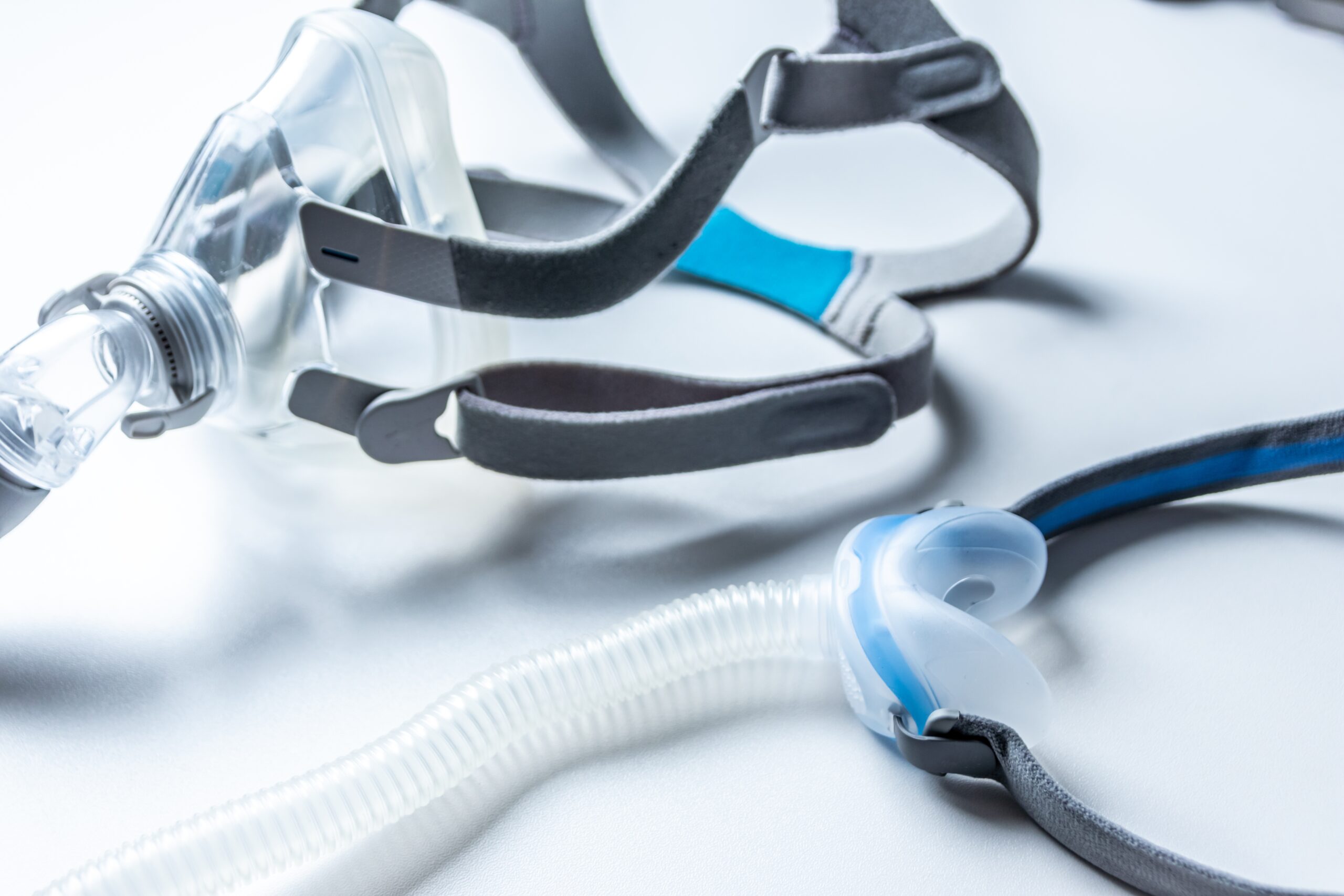
Cleaning your CPAP machine can feel like a hassle, but it’s worth the effort. Regularly washing the mask, hose, and other components of your CPAP machine by hand prevents harmful bacteria, viruses, and fungi from growing in them.
Over the last several years, a number of CPAP cleaning devices that claim to efficiently and effectively sanitize CPAP components have come onto the market. However, the U.S. Food and Drug Administration (FDA) has yet to authorize any of these products. In fact, the FDA cautions against using them.
Think You May Have Sleep Apnea? Try an At-Home Test

our partner at sleepdoctor.com
Save 45% on your Sleep Test Today
Shop now“Truly grateful for this home sleep test. Fair pricing and improved my sleep!”
Dawn G. – Verified Tester
Why CPAP Machines Need to Be Cleaned
Continuous positive airway pressure (CPAP) machines help treat obstructive sleep apnea (OSA) by pumping pressurized air from a machine through a hose and into a mask covering the nose and mouth. The pressurized air prevents the narrowing or collapse of soft tissues in the throat, keeping the airway open.
CPAP machines need regular cleaning to remove germs, dead skin cells, and oils that accumulate in and on the hose, mask, headstraps, and other components. Not cleaning your CPAP machine often enough can have consequences, including:
- Reinfecting yourself with an illness, such as a cold
- Getting a bacterial or fungal infection
- Developing skin irritation
- Accelerating the breakdown of mask components and affecting its seal
To avoid these problems, stick to a regular cleaning schedule for your CPAP machine (see below for more details on how and when).
Why CPAP Cleaning Machines Aren't FDA-Approved
In recent years, companies have started selling machines that promise to sanitize CPAP equipment more quickly or thoroughly than traditional hand-washing. These devices often use ozone gas or ultraviolet (UV) light and come in the form of enclosed boxes or bags that claim to clean and disinfect CPAP masks, tubing, and other components.
However, the FDA hasn't approved or cleared any of these machines for CPAP cleaning. Ongoing research and reported adverse events have raised safety concerns. As a result, the FDA advises CPAP users not to use ozone or UV light-based cleaners until their safety and effectiveness have been properly evaluated.
It’s also important to note that these machines do not remove visible dirt, oil, or residue from equipment surfaces. Even when used, hand-washing is still required — meaning these devices add time and cost without replacing standard cleaning.
In addition to offering limited practical benefit, ozone and UV-based CPAP cleaning products may expose users to harmful levels of ozone gas or UV radiation, prompting the FDA to issue warnings about potential health risks.
Ozone Gas Cleaners and Sanitizers
Ozone is a gas consisting of three oxygen atoms. While it occurs naturally in the atmosphere and is used in some industrial and medical settings to disinfect surfaces and water, ozone is also a known air pollutant and can be harmful to human health.
The concentration of ozone needed to sanitize CPAP equipment may pose health risks. Manufacturers of ozone-based CPAP cleaners and sanitizers often claim their devices contain the gas within a sealed chamber to prevent user exposure. Still, they caution that treated equipment must be properly aired out after cleaning, as residual ozone can remain trapped inside masks or tubing.
But the FDA has found that ozone can leak from CPAP cleaning devices, creating the potential for unsafe levels of ozone. The FDA has also tested some of these products by running them in small bathrooms. For several models, ozone levels within the room have exceeded safety standards following device operation.
Moreover, the FDA has discovered that ozone can stay in CPAP tubing for hours after cleaning if fresh air isn't circulated through it. This means that users could unknowingly inhale ozone after cleaning their CPAP machines, and this exposure can lead to symptoms, including:
- Headaches
- Coughing
- Asthma attacks
- Trouble breathing
- Sinus irritation
Exposure to ozone from these devices could increase a person’s risk of getting a respiratory illness or aggravate chronic conditions like asthma.
UV Light Cleaners and Sanitizers
UV light is naturally produced by the sun and artificially produced by certain types of lighting. Some at-home systems use UV light to purify drinking water, and in medical facilities, UV lamps have long been used to disinfect surfaces.
But as anyone who has ever spent too much time in the sun knows, directly exposing the skin to UV light can cause irritation and even burns. Most CPAP cleaning devices that use UV light automatically turn off the light when the door of the device is open, but the FDA has not verified that this provides sufficient protection from UV exposure.
Risks Associated With UV Light CPAP Cleaning Devices
There haven't yet been any reports of UV light from CPAP cleaning devices causing injury. That said, the FDA warns that inadvertent exposure to UV light from these products has the potential to harm the eyes or skin and even elevate the risk of skin cancer.
Additionally, the UV light generated by these devices may not reach the interior surfaces of CPAP accessories, such as the hoses. As a result, germs could remain, putting the user at risk of an illness or infection.
How to Clean Your CPAP Machine
When it comes to cleaning your CPAP machine, it's best to refer to your device’s instructions. Most manufacturers recommend washing the CPAP components by hand with mild soap. You may also use one part white vinegar diluted with three to four parts water, which can help reduce odors.
Never use cleaning products that leave a residue or contain harsh chemicals like bleach. Do not boil CPAP parts or clean them in a washing machine unless instructed to do so by the manufacturer. And make sure to unplug your machine before cleaning it.
While cleaning instructions may vary by model or manufacturer, there are some recommended practices that are similar across most devices.
| CPAP Component | Recommended Cleaning Frequency | Cleaning Instructions |
|---|---|---|
| Nose pillows or cushions | Daily |
|
| Mask | Every other day |
|
| Headgear and chin strap | Weekly |
|
| Hose or tubing | Weekly |
|
| Reusable foam filters | Weekly |
|
| Humidifier chamber | Weekly |
|
| CPAP machine | As needed |
|
In addition to following these instructions, you can discourage the growth of germs by disconnecting and hanging up the tubing of your CPAP machine on the mornings you don’t wash it. Also, consider using distilled water in the device’s humidifier, which will limit mineral buildup.
When in doubt about how to clean your device, contact your CPAP machine’s manufacturer or a healthcare provider.
Additionally, remember to check your CPAP supply replacement schedule, which is typically provided by your CPAP machine’s manufacturer. This schedule generally recommends replacing CPAP parts—such as the hose, mask, mask cushion, filter, and humidifier chamber—every one to six months.
If you have questions about reimbursement for CPAP supplies, you can contact Medicare, your insurance provider, or a durable medical equipment provider for more information.
Reporting a Problem With a CPAP Device
The FDA collects information about adverse events related to medical devices, including CPAP equipment. If you experience an issue with your CPAP machine or a CPAP cleaning device, consider reporting this issue to FDA’s MedWatch Online Voluntary Reporting Form.