Medical Disclaimer: This content is for informational purposes and does not constitute medical advice. Please consult a health care provider prior to starting a new treatment or making changes to your treatment plan.
In June of 2021, Philips, a major manufacturer of devices to treat sleep apnea, announced a recall affecting around four million of its products that were determined to pose a potential health risk to people using them.
Specifically, Philips identified potential problems with a piece of foam inside of these devices. After reporting this information to the FDA, Philips initiated a product recall of nearly 20 different models, including commonly used continuous positive airway pressure (CPAP) machines.
As of April 2024, Philips Respironics was ordered to halt all production and sales of devices in the U.S. According to federal officials, an agreement has been reached to overhaul Philips’ safety inspection process over the next five years. Philips has now refurbished and replaced around 90% of the defective devices, but all of these machines will now require additional testing and review.
To help provide the important details about the Philips CPAP recall, we cover why it happened, what owners of a recalled device should do, and the process for getting a replacement device.
Which Phillips CPAP Machines Are Recalled?
The Philips Respironics recall includes a wide range of products, including multiple CPAP, BiPAP, ASV, and ventilator models manufactured before April 2021.
For any specific device, finding and locating the serial number will verify the model and the date it was manufactured, and this information can be used to confirm whether that machine is involved in the recall.
CPAP Devices
Continuous positive airway pressure (CPAP) devices support steady breathing during sleep by sending a consistent stream of pressurized air into the upper airway.
Recalled CPAP models include:
- DreamStation CPAP
- DreamStation GO CPAP
- Dorma 400
- Dorma 500
- System One 50 Series
- System One 60 Series
- RemStar SE Auto
The recall also includes many auto-CPAPs, commonly known as APAP devices, that are part of these same product lines. APAP machines are similar to CPAPs, but they can adjust the air pressure level based on a person’s breathing patterns during sleep.
BiPAP Devices
A number of recalled devices are bilevel positive airway pressure (BiPAP or BPAP) machines. A BiPAP also sends pressurized air into the airway, but it is distinct from a CPAP machine because the air pressure level is different for inhalation and exhalation.
Models of Philips BiPAP machines involved in the recall include:
- A-Series BiPAP A30
- A-Series BiPAP Hybrid A30
- A-Series BiPAP A40
- A-Series BiPAP V30 Auto
- C-Series S/T AVAPS
- C-Series S/T
- C-Series System One BiPAP AVAPS
- C-Series System One BiPAP S/T
- DreamStation BiPAP
- DreamStation BiPAP AVAPS
- DreamStation BiPAP S/T
- DreamStation BiPAP autoSV
- System One BiPAP autoSV
- System One BiPAP autoSV Advanced
- System One 50 Series
- System One 60 Series
- OmniLab Advanced+
ASV Devices
Adaptive servo-ventilation (ASV) devices track a person’s breathing and can adjust the air pressure during inhalation or exhalation in order to resolve breathing disruptions.
ASV devices that are part of the Philips recall include:
- DreamStation ASV
- DreamStation S/T
- DreamStation AVAPS
- C-Series ASV
- System One ASV4
Ventilators
A ventilator can provide breathing assistance to people with many conditions while they are awake, which is different from CPAP, BiPAP, and ASV devices that are more frequently used for breathing problems during sleep.
Philips ventilator models that have been recalled include:
- E30
- Garbin Plus, Aeris, LifeVent
- Trilogy 100
- Trilogy 200
Why Philips CPAP Devices Are Recalled
Philips recalled CPAP, BiPAP, ASV, and ventilator products because one component inside the machines was discovered to have the potential to be defective and harmful.
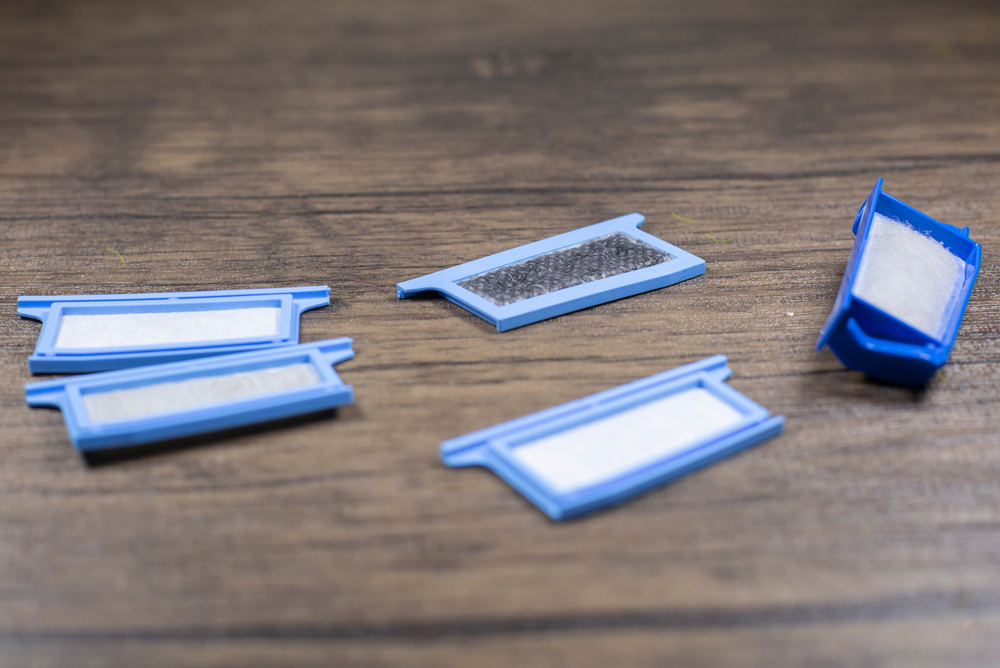
In the affected devices, a piece of foam was used to help reduce the sound generated by the machine. This foam, known as polyester-based polyurethane (PE-PUR), was found to be at risk of breaking down, which could pose health risks to users of the devices.
An investigation had found that Philips withheld thousands of complaints about foam issues for over a decade before warning its customers in 2021. Philips has recalled more than 5 million of the machines since then, and is now prohibited from selling sleep apnea devices and other respiratory machines in the U.S.
Health Risks of Using Recalled Machines
A range of potential health problems could arise in people who use recalled Philips devices. There are two main reasons why it is believed that a breakdown of the PE-PUR foam in these devices could create health risks.
- Inhalation or ingestion of foam particles: If the foam starts to disintegrate, tiny pieces may be carried into the nose or mouth, where they may be swallowed into the stomach or breathed into the lungs.
- Breathing in chemicals emitted by foam: The PE-PUR foam may give off small amounts of chemicals known as volatile organic compounds (VOCs) that may contribute to certain health issues when inhaled.
Possible symptoms from these exposures to the PE-PUR foam include:
- Headache
- Skin irritation
- Eye irritation
- Irritation of the nose or respiratory tract
- Sinus infections
- Cough
- Asthma
- Nausea and vomiting
- Allergies
- Dizziness
- A feeling of pressure in the chest
To date, no evidence has been found that links the use of recalled machines to a higher risk of cancer, but the possibility of carcinogenic effects cannot be ruled out until further long-term research is concluded.
What to Do if Your Philips CPAP Is Recalled
If you have a Philips CPAP machine or other device that is part of the recall, avoid opening it or doing anything to change or modify its components, including the PE-PUR foam. Immediately tell your doctor if you have any symptoms. Your doctor can evaluate those symptoms and suggest steps to address them. Your doctor can also file a report with the FDA.
In addition, your doctor can talk with you in detail about your options. In many cases, it is advisable to seek out a new machine from a different manufacturer or contact Philips for an FDA-approved replacement.
If you decide to seek a replacement device from Philips, you will need to follow several steps.
- Find the model and serial number: Your machine should have a label that shows the model name and the serial number. Locating this information is necessary to initiate the process of getting a replacement from Philips.
- Register your device: Once you have the serial number, you can register your machine, which puts you on the list to get a new device shipped to you.
- Watch for email updates: Before Philips sends you a new machine, you will receive an email with further details. Keep an eye out for this message, and when it arrives, confirm all included information. In the meantime, you can follow the Philips page about the recall to stay updated with any new information that is released.
- Return your old device: After your new machine arrives, you will be instructed to send back your old machine. Philips covers the costs for shipping back the recalled device.
There is no set timeline for receiving a replacement machine, but the Philips patient portal may offer updates about the expected shipping date. A prioritization program is available if you have a documented medical need to get a new device more quickly.
Are Replacement Machines Safe?
Replacement devices offered by Philips have been refurbished with a silicone foam in place of the potentially hazardous PE-PUR foam. Testing by Philips has generally shown this silicone foam to be safe, but more independent testing is required to clearly demonstrate its safety.
Until that testing is concluded, the FDA recommends continuing to use replacement devices rather than going without treatment for breathing problems. If you have questions or concerns about the safety of a replacement device or other potential treatments, you should consult with your doctor or a sleep specialist.
Considering Other Sleep Apnea Treatments
While CPAPs and other PAP devices are typically effective in treating sleep apnea, they are not the only treatment options. Alternatives to PAP therapy may be a consideration if you do not want to continue using a recalled Philips device nor seek out a replacement machine.
The options for sleep apnea treatment can vary based on a number of factors, such as the severity of your symptoms, your overall health, and the type of sleep apnea that you have.
For obstructive sleep apnea, which is the most common type, alternative treatment options may include:
- Wearing a custom-fitted device in your mouth during sleep to help hold your tongue or jaw in a position that keeps your airway open
- Having surgery to remove or reconstruct tissue that can block the airway while you sleep
- Having an operation to implant a device in your chest that delivers electrical stimulation to a nerve that affects tissues near the airway
Depending on your circumstances, these treatments may not be available or beneficial. For that reason, it is important to talk to your doctor about each of your treatment options and their potential benefits and drawbacks.